Research
We study how lipid homeostasis and protein lipidation regulate the organization and function of cellular membranes in health and disease. Pathogens exploit host membranes to replicate and spread, while host cells evolve mechanisms to detect and counteract these perturbations. By dissecting these interactions, we uncover fundamental rules of membrane biology and identify new opportunities for therapeutic intervention.
Our work combines biochemical assays, structural analysis (e.g., cryo-ET), high-resolution imaging, and quantitative proteomics/lipidomics, applied across diverse infection models with a current focus on SARS-CoV-2.
A unifying theme is S-acylation, the reversible attachment of fatty acids to proteins. This modification controls membrane dynamics throughout biology and plays a central role in human health and disease. From this perspective, we address various questions such as:
How does S-acylation of viral proteins shape viral membranes and influence replication?
How is host lipidation remodeled during infection, and what are the consequences for immunity?
How do cells detect membrane lipid damage and convert it into innate immune defense?
(check our recent contribution to review the fundamentals of S-acylation)

Viral protein lipidation
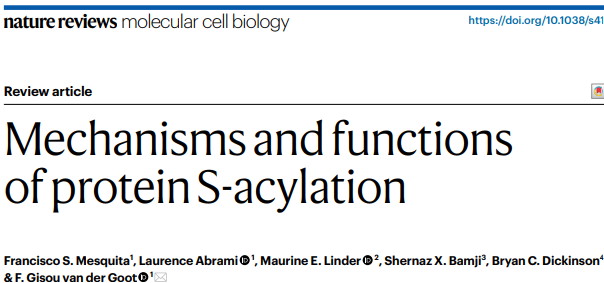
We showed that S-acylation of viral proteins, particularly the Spike glycoprotein, alters virion lipid composition and enhances infectivity. This opens fundamental questions on how lipidation controls viral envelopes, and broader host–virus interactions. We are now expanding this work to other viral and host proteins to uncover principles of membrane remodeling during infection.
Host lipidation
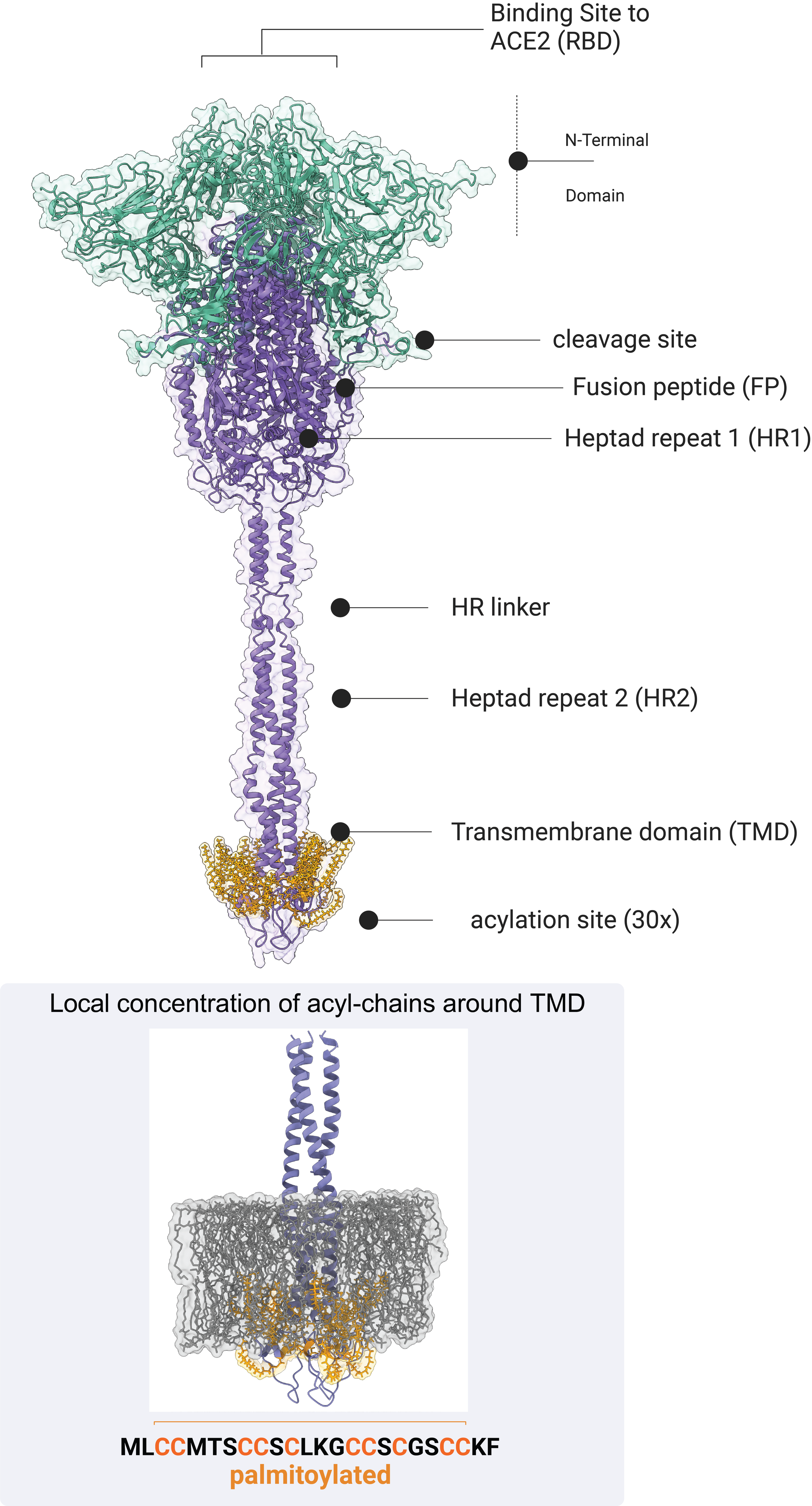
We mapped how host lipidation is rewired during infection (volcano plot - manuscript in preparation) and found that blocking depalmitoylation enzymes can reduce SARS-CoV-2 infection. The next step is to detail the main host proteins involved, define their roles in infection and immunity, and explore how lipidation integrates with innate immune regulation. Our lab is now focusing on key targets to uncover how these processes shape immunity and disease.
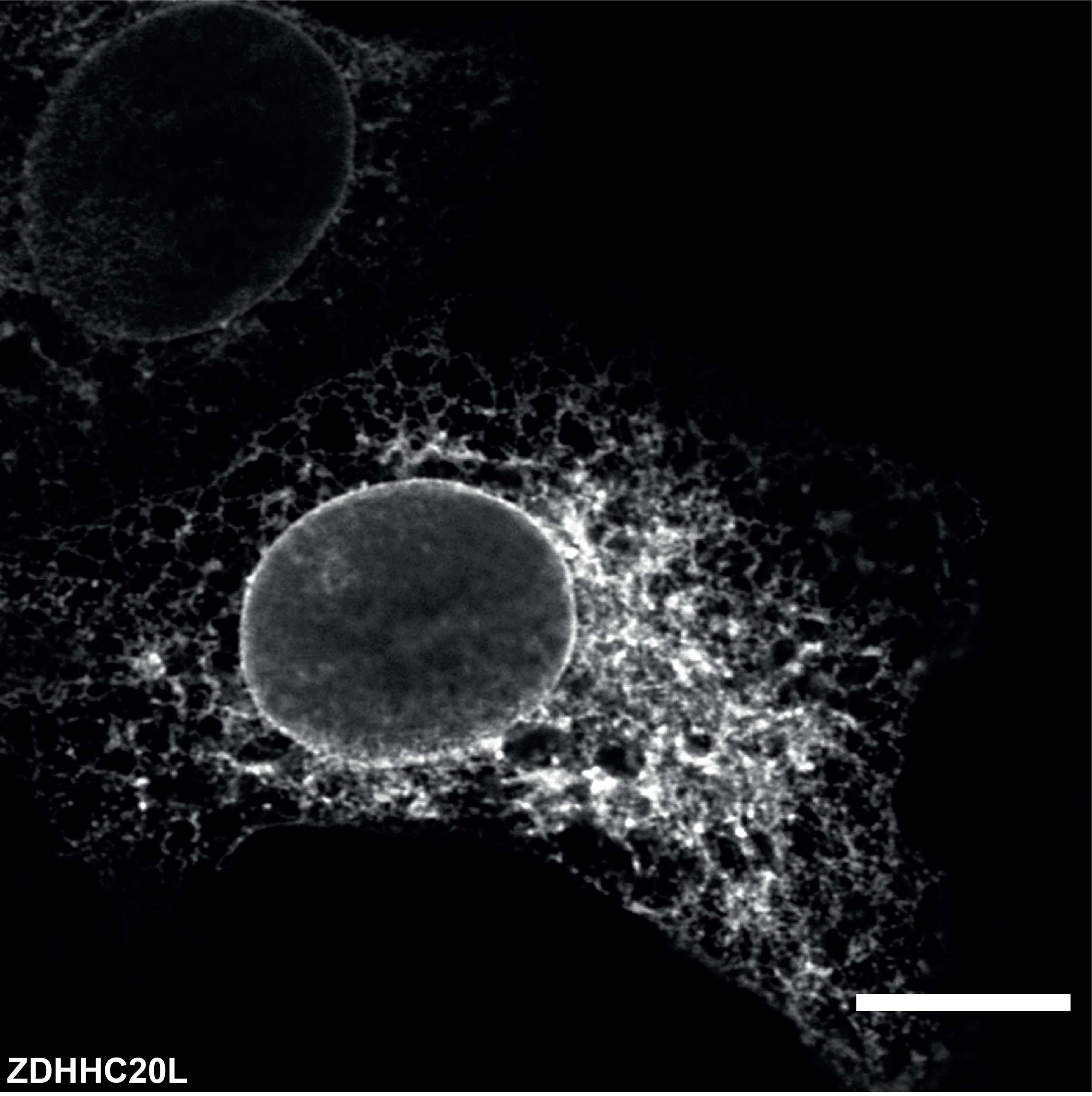
Lipid oxidation damage & innate immunity
We discovered that ZDHHC20, a major acyltransferase that modifies viral proteins from pathogens such as SARS, HIV, and influenza, is transcriptionally regulated during infection.
SARS-CoV-2 hijacks this regulation to maintain its lipid composition and infectivity. This study uncovered a previously unknown transcription regulatory mechanism, also triggered by bacterial toxins and colitis in vivo.
In our most recent preprint, we describe this lipid oxidative radical damage (LORD) pathway, which links lipid-Reactive Oxygen Species (ROS) stress to innate immunity.
The “LORD pathway” is centered in ZNF354A, a transcriptional repressor that responds to ROS accumulation by releasing specific DNA sites (Chip-seq figure) that promote cellular antioxidant capacity. This new circuit reveals new regulators and effectors critical for overcoming oxidative stress and infection, raising the question of how these processes are tuned by protein acylation. We are continuing to explore this with collaborators, investigating its implications across infection, inflammation, and redox biology.